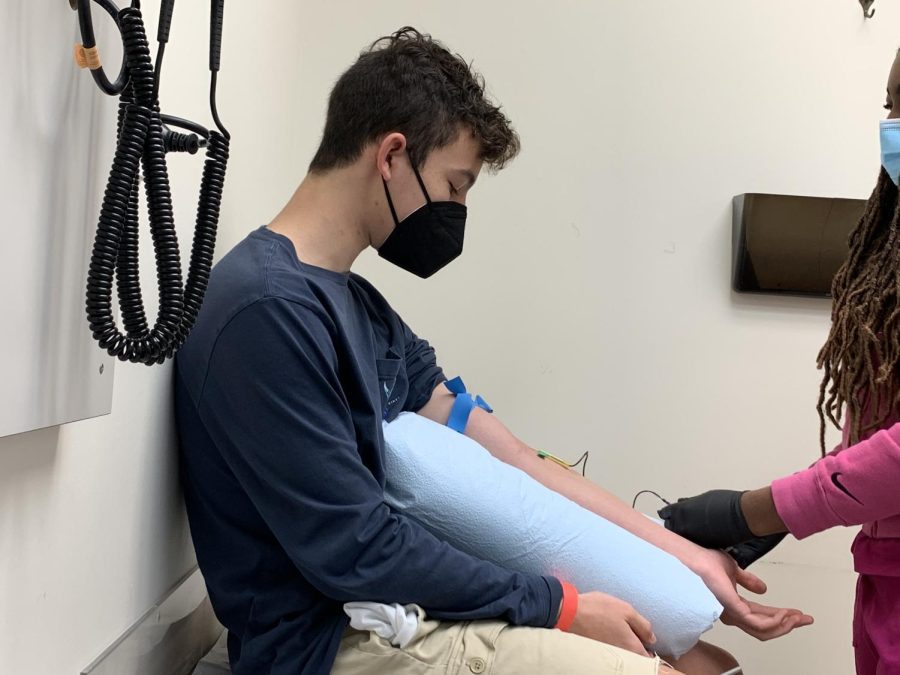
Junior Gabe Foy participates in COVID-19 vaccine trials
Junior Gabe Foy’s blood is taken to check for the prevalence of antibodies from the vaccine.
As the eldest of five siblings, the pandemic was hard for CVHS Junior Gabe Foy. Having to help his siblings with technology made it extremely difficult to focus in class. His house was also filled with many voices, impacting how he could speak and participate in class. But with both his parents in the medical field, he knew a vaccine would be the quickest path out of lockdown. That’s why he jumped at the opportunity to be a part of a 13-month-long vaccine trial.
In January, Foy participated in the Moderna vaccine trials for 12-17-year-olds. Previous trials and the FDA’s emergency use authorization of the vaccine had only been available for adults. Houston was one of 20 cities to participate in Moderna’s trials for adolescents.
As doctors, both of Foy’s parents got vaccinated as soon as they could. When his mother told him about the Moderna trial, he was eager to sign up.
“So there was money involved in the trial. That was one factor, but also there was also the motivation to get the vaccine as soon as possible,” Foy said.
Beyond the difficulties with online learning, the pandemic had hindered Foy’s ability to visit his relatives.
“The time I spent with family outside the house was extremely limited as I could only see them during breaks, and there were many requirements to travel to Puerto Rico where a lot of my family lives,” Foy said.
The chance to regain normalcy by getting vaccinated earlier than the national rollout, along with the $700 compensation, was also attractive to the hundreds of other Houston teens who participated. While he wasn’t able to interact with them, Foy wasn’t alone. His 13-year-old sister joined him, and he even recruited others.
“When I told my friends that I was doing the trials, they were actually glad that I was doing it, and one of my other friends actually participated too after he heard that I was doing it,” Foy said.
This was the first time that Foy had been a part of a clinical trial. After signing a contract, he was given some preliminary information before the first day.
“I was kind of nervous because I didn’t know what to expect,” Foy said.
On January 8, Foy walked into the clinic hosting the trial. Before the shot, he got his blood drawn and a COVID vaccination test. Participants had a 2-to-1 chance of receiving the vaccine over the placebo, and the clinic informed him that he would be receiving the former. As the shot was administered, nothing was running through his mind.
“I was just focusing on looking away from the needle-like I always do when I get a shot,” Foy said.
Side effects of the COVID vaccine are common, and it was one of the factors monitored in the trial.
“When I first got the shot, I felt pretty tired,” Foy said. “I actually got a fever the next day, a low fever, like 99 or 100. It wasn’t too bad. But the day after, I felt perfectly fine.”
After the shot, Foy underwent an antibody test but spent the remainder of the day normally. A month later, on February 5th, he returned for his second shot. Additionally, every two months, six times over the 13-month-period, he returned for follow-up visits. His last visit will be in February.
“They’re still monitoring. I went to my last appointment about three weeks ago. And they just ran more blood tests and did a COVID test,” Foy said.
The researchers running the trials also collected data by sending him questionnaires via an app called Medidata. Additionally, they’re interested in performing further trials in the future.
“There’s another upcoming trial for booster shots, and they want to see if I’m interested,” Foy said. “I’m likely to participate in it.”
As far as his future, Foy is still unsure about his career path. However, participating in the trials has given him some guidance.
“Looking back at the vaccination trial, I find the medical field really interesting, and I’m part of the Future Medical Leaders of America club,” Foy said.
Naturally, as a participant in the trials, Foy is a proponent of vaccines. He feels that the vaccine administration has been relatively successful in the U.S., barring the individuals who are “not willing to change their perceptions.”
“I don’t think it’s very smart not to get vaccinated,” Foy said.
As of October 16th, 66% of eligible individuals (12 years old and older) in Houston are fully vaccinated. Despite the divisive stances on vaccines in the country, being a part of the trials has given Foy insight. He got a glimpse into how society has banded together in the wake of the virus.
“It has actually given me a new perspective to how many people are willing to bring an end to COVID, and this is supported by all the vaccination efforts that we can see at the trial. There’s a lot of people that actually went to the trial,” Foy said. “The people there at the trial were really thankful for me participating, along with my sister.”
Your donation will support the student journalists of Carnegie Vanguard High School. Your contribution will allow us to cover our annual website hosting costs and fund field trips, competition fees, and equipment. We appreciate your support!

Hi, I'm Jessica! I am a senior and one of the Feature Editors for Upstream News. I love dance, Marvel, and cats.