Editorial: COVID19 TRIPS waiver and the hypocrisy of American pharmaceuticals
While many wealthy nations have returned to pre-pandemic life, vaccine desert-countries continue to struggle with containing COVID19.
In December 2020, the FDA issued an Emergency Use Authorization (EUA) for the Pfizer COVID vaccine; the Moderna and Janssen vaccines were issued EUAs shortly after. In May, the CDC announced that fully vaccinated people could participate in indoor and outdoor activities without wearing masks. By July, the country seemed as though it returned back to pre-pandemic life. I visited relatives after skipping holiday and birthday dinners and had weekly pho-and-movie nights with friends; for the first time in over a year, I was not sitting in my room for more than half the day.
Meanwhile, on the other side of the world, my family in Vietnam went into lockdown; neither outdoor activities nor gatherings of more than two people were allowed and people could be fined up to 3 million VND (130 USD) for going outside without a valid reason. While Vietnam received praise earlier in the year for how they contained the virus, the Delta variant wreaked havoc in the country. Like most less wealthy nations, Vietnam does not make its own vaccines; the “recipes” for COVID-19 vaccines are hidden by intellectual property protections.
The Trade-Related Aspects of Intellectual Property Rights (TRIPS Agreement), signed by member states of the World Trade Organization (WTO) in 1994, is at the center of vaccine patent protection and suspending it. Specific to this case, the TRIPS Agreement protects intellectual property rights for technologies needed to prevent or halt the spread of COVID19 (TRIPS Part II, Sections 1, 4, 5, and 7); this includes vaccines and vaccine technologies.
In October 2020, South Africa and India sent a proposal to the WTO to temporarily hold off on the TRIPS “until widespread vaccination is in place globally, and the majority of the world’s population has developed immunity.” However, that proposal was rejected due to opposition from high-income countries like the United States and the European Union. On May 5, President Biden announced that he and his administration would support waving patent protection for COVID19 vaccines. The eight top Pfizer and Moderna shareholders, whose intellectual property is protected by the TRIPS Agreement made over $10 billion last week when their stock holdings skyrocketed after the discovery of the new Omicron variant.
Despite the WHO and Biden Administration’s urges to pharmaceutical companies, including Pfizer and Moderna, to license their vaccine-related technology to third-party manufacturers, to give low- and middle-income countries access to vaccines, these companies have chosen not to do so. Moderna spokeswoman Colleen Hussey said that the company is “willing to license our intellectual property for Covid-19 vaccines to others for the post-pandemic period.” These are the same companies who believe “that all individuals, everywhere, deserve access to quality healthcare and the opportunity to lead healthy lives.” To me, it seems as though those in other less wealthy nations are not included in Pfizer’s definition of “everywhere”.
Some nations in the WTO, including Angela Merkel, chancellor of Germany, argue that lower-income nations would not be able to produce COVID19 vaccines even if they were given the formula. These nations lack the infrastructure, the knowledge, and the money. The Pfizer and Moderna vaccines are mRNA vaccines. This means two things: it’s more expensive to manufacture in comparison to vector-based vaccines and it requires in-depth knowledge of mRNA technology. In addition to being expensive to produce, vaccines must be stored at temperatures up to -70 degrees Celsius. Currently, the WHO’s tech transfer hub in South Africa is trying to replicate Moderna’s vaccine recipe. “If we had Moderna or BioNTech with us, we could get to an approved vaccine in 18 months, but without them, we have to go through full development — so it’s 36 months if everything goes perfectly, but it could be longer,” said the head of the WHO’s Initiative for Vaccine Research Dr. Friede to the New York Times. The pharmaceuticals have the ability to assist contract manufacturers with receiving equipment and intellectual property. They’re just choosing not to.
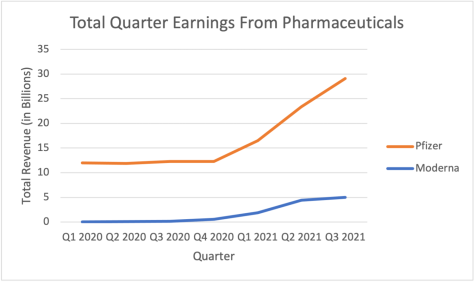 Within the Biden Administration, there is some opposition in waving TRIPS as a result of increased new competition. Moderna and Pfizer combined are projected to earn over $53 billion in revenue this year. Part of this is because TRIPS blocks competitors. Many also fear that the introduction of competitors would deter pharmaceuticals and innovators from researching and developing in the future; prospected mRNA vaccines include those for cancer and HIV/AIDS. However, Moderna was part of Operation Warp Speed, which gave the company $2.5 billion from the government and access to technology developed by the National Institutes of Health. Additionally, while Pfizer did not take federal money for research, the Trump Administration in July 2020 agreed to buy at least $1.95 billion worth of vaccines from the company; this revenue most likely acted as an incentive for the company to research and develop vaccines to combat the spread of COVID19. Since the money these companies received was from taxpayers, isn’t it unethical for the company to withhold its vaccine information? To me, it looks like these companies only stepped up to develop vaccines for COVID19 because the endeavor is highly profitable.
Within the Biden Administration, there is some opposition in waving TRIPS as a result of increased new competition. Moderna and Pfizer combined are projected to earn over $53 billion in revenue this year. Part of this is because TRIPS blocks competitors. Many also fear that the introduction of competitors would deter pharmaceuticals and innovators from researching and developing in the future; prospected mRNA vaccines include those for cancer and HIV/AIDS. However, Moderna was part of Operation Warp Speed, which gave the company $2.5 billion from the government and access to technology developed by the National Institutes of Health. Additionally, while Pfizer did not take federal money for research, the Trump Administration in July 2020 agreed to buy at least $1.95 billion worth of vaccines from the company; this revenue most likely acted as an incentive for the company to research and develop vaccines to combat the spread of COVID19. Since the money these companies received was from taxpayers, isn’t it unethical for the company to withhold its vaccine information? To me, it looks like these companies only stepped up to develop vaccines for COVID19 because the endeavor is highly profitable.
A contributing factor to the low vaccination rate in lower-income countries is their inability to produce vaccines. London analytics company Airfinity reported that of the 1.5 billion vaccines produced in October of this year, nearly 60% went to high-income countries, including the UK, nations in the EU, the US, and Canada. The company estimates that up to half the vaccines purchased by these countries are going unused.
As part of the Biden Administration’s commitment to aiding low-income countries, these countries, including Vietnam, have been receiving a portion of the US’s excess vaccines. However, there are still unused vaccines in the US and in other high-income nations. Rasmus Bech Hansen, CEO of Airfinity, said to NPR that the “surplus would have been enough to meet a key goal for this year set by the World Health Organization: vaccinating at least 40% of people in lower-income countries.” Temporarily suspending patent protection for the COVID19 vaccine is a vital first step in helping less wealthy nations get access to vaccines. The spread of infrastructure should come next.
Vietnam, after three months of strict restrictions, lifted its lockdown at the end of September. My family in Vietnam is now vaccinated. However, they received their doses almost eight months after everyone in my family living in the US. In fact, I received my booster shot before most of my family in Vietnam was fully vaccinated. In this time, COVID19 cases in Vietnam continued to spike; the country continues to reach new case records, with November 29’s 17,040 7-day rolling average becoming Vietnam’s highest since the start of the pandemic. While the percentage of people who have received at least one dose of a COVID19 vaccine is rapidly increasing in Vietnam, going from 66.44% on November 14 to 72.5% on November 29, the same increase in vaccination rates cannot be seen in other countries. In the same time period, the percent of people at least partially vaccinated in low-income nations went from 4.7% to 6% while that of high-income nations increased from 72.9 to 74.2%.
The Omicron variant is the newest addition to the COVID19 variants, and we should continue to expect more in the future; the virus will continue to mutate as it spreads among vaccine deserts in poorer nations. “The current variant, Omicron, is the result of the world’s failure to vaccinate its citizens in an equitable and efficient manner,” said Dr. Ayoade Alakija to Time Magazine. If pharmaceutical companies really stand behind their mission statements, they need to waive their intellectual property and give others access to patent-protected COVID19 vaccine development processes. Until then, I believe that these pharmaceuticals do not have the right to call themselves health care companies.
Your donation will support the student journalists of Carnegie Vanguard High School. Your contribution will allow us to cover our annual website hosting costs and fund field trips, competition fees, and equipment. We appreciate your support!












Ava Lim • Dec 9, 2021 at 1:46 pm
I like how you set up fact against fact to back up your argument. The personal aspect to your story also your argument more convincing, as well as humanizing an issue that’s characterized heavily by numbers.